
Calculating Temperatures Under Hood of a Prebake Anode Cell
Marc Dupuis GéniSim Inc. 3111 Alger St., Jonquière, Québec, Canada G7S 2M9
Warren Haupin 2820 Seventh Street Road Lower Burrell, PA 15068 USA
ABSTRACT
In computer modeling of a prebaked anode Hall Héroult cell, one needs to know the temperature of the mixture of air and cell gas (Tairin) that cools the top of the cell crust and the anode rods under the cell hoods. This temperature can be measured, of course, if a similar operating cell is available, but not if the model is of a distinctly new design. However, it is also possible to estimate the temperature. This is the aim of the present work.
First we must calculate the air drawn in under the hoods at potroom temperature, and combine it with the CO2 and CO escaping at electrolyte temperature to produce a gas blend at temperature, Tblend. The CO of the mixture burns, generates heat, forms more CO2, and consumes O2 from the air drawn in. Also, heat is generated by air burning of the anode forming additional CO2 and consuming O2. The heats of combustion of CO and air burning of anode carbon plus the heat from the cover and anode stubs raise the temperature of the mixture of gases to temperature, Tmix.
This gas mixture then extracts heat from the anode rods and rises to the exhaust temperature, Texh. In order to compute Tblend, Tmix and Texh, the heat capacities of the gas mixtures is needed. Finally, Tairin is calculated as the log mean of Tmix and Texh, just as the log mean of inlet and outlet temperatures are used to calculate heat transfer in heat exchangers.
DEFINITION OF TERMS

ASSUMPTIONS
It is assumed the cell is so designed that the air is drawn in uniformly around the periphery of the cell, that it mixes uniformly with the cell gas producing a gas mixture at an initial temperature, Tblend. The CO of this gas mixture burns to CO2 at temperature, Tblend, consuming some of the oxygen and generating heat, Qcombust. Also some of the oxygen of this mixture air burns the tops of the anodes generating CO2 at temperature Tblend and generates heat, Qairburn. The combination of Qcombust, Qairburn and Qtop heats this gas mixture raising its temperature to Tmix. As this gas rises, it extracts heat from the anode rods raising the gas temperature to Texh. This gas mixture then exits uniformly into a slotted gas collection duct extending the length of the cell at the top of the superstructure. Tairin is taken as the log mean of Tmix and Texh.
CALCULATIONS

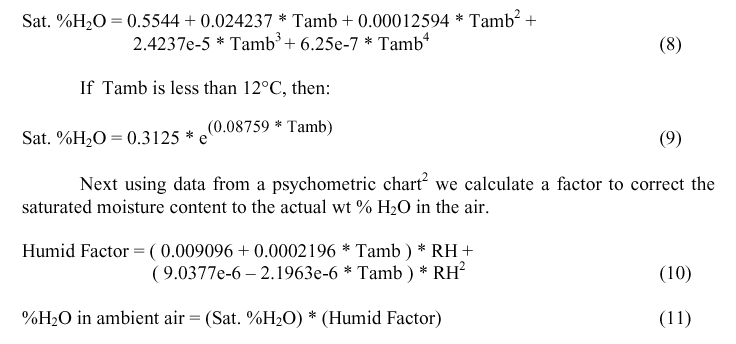
The moisture content of the air drawn in has a significant effect on the heat capacity of the air. To get the percent moisture in the ambient air first we calculate the mass % water in water saturated air2 based on the temperature of the air in the potroom.
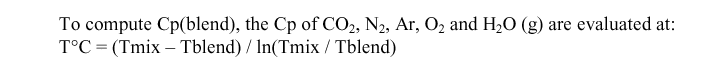
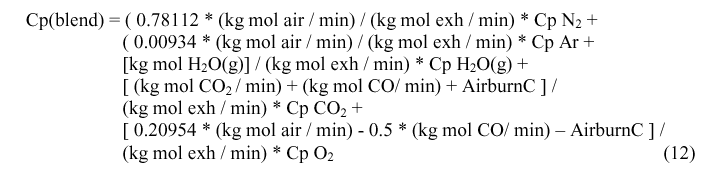
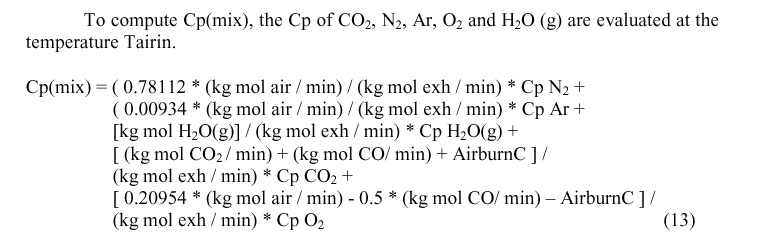
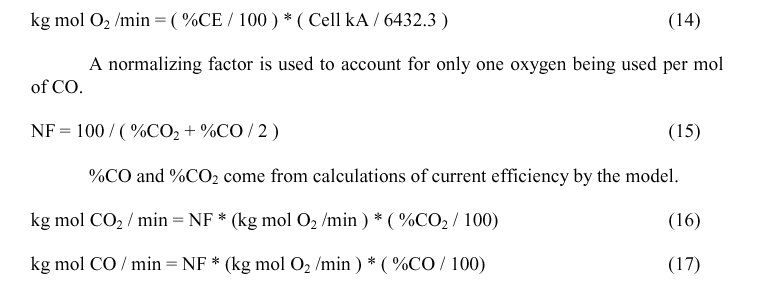
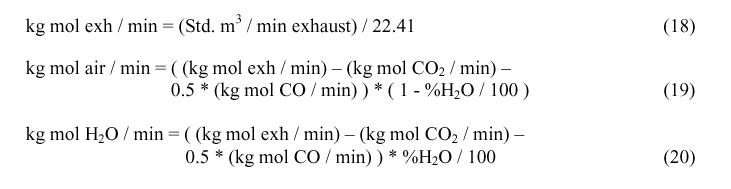

Calculating Qcombust
Qcombust, the quantity of heat generated by the combustion of CO as it exits the electrolyte is needed to calculate Tmix. Qcombust is obtained by calculating the enthalpy of the reaction:

Calculating Qairburn
We take the total carbon consumption by electrolysis and multiply it by a fraction representing the amount that air burns to get the airburn carbon. Net carbon consumption will be the sum of electrolytically consumed Carbon plus AirburnC plus Dusting.

Calculating Tmix
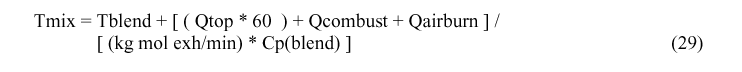
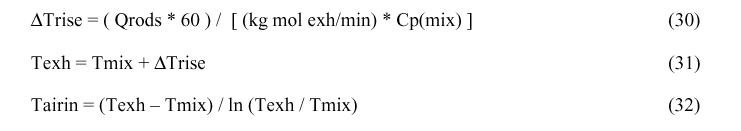
Because the calculation of many of the values depend upon Tblend, Tmix and Tairin, not yet calculated, the solution has to be an iterative process. The suggested value for first iteration is Tblend = 50° C, Tmix = 160° C and Tairin = 170° C.
ANALYSIS OF RESULTS
Figure 1 shows, as expected that the temperature under the hood will drop as more air is drawn in.
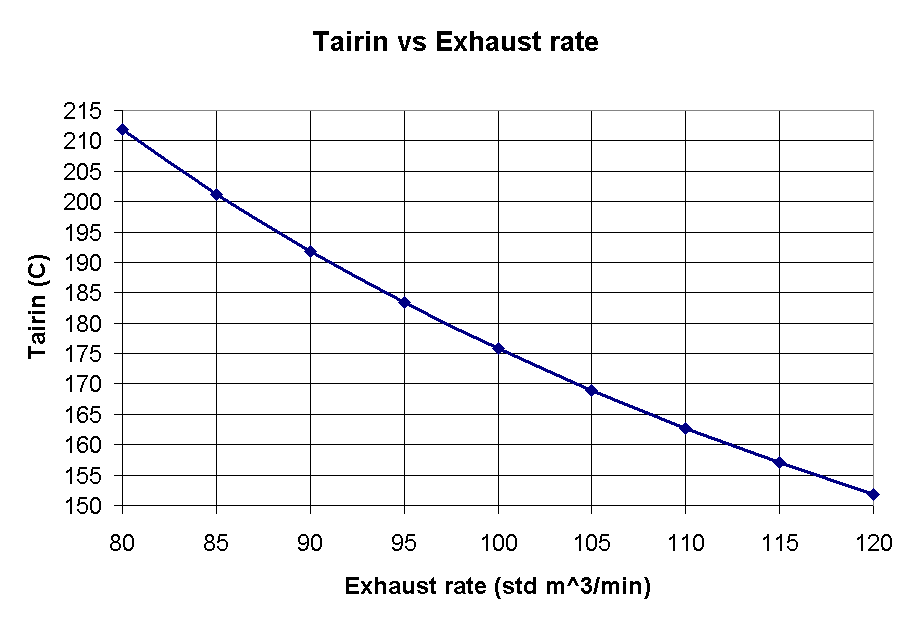
Figure 1 - Tairin vs Exhaust rate
Figure 2 indicates that as current efficiency increases, the temperature under the hood should drop. This is an idealized curve and may not be seen in practice. It is true that more energy goes into making aluminum and less into heat at higher current efficiencies. Changes in crust cover and anode changing will often obscure the effect of current efficiency.
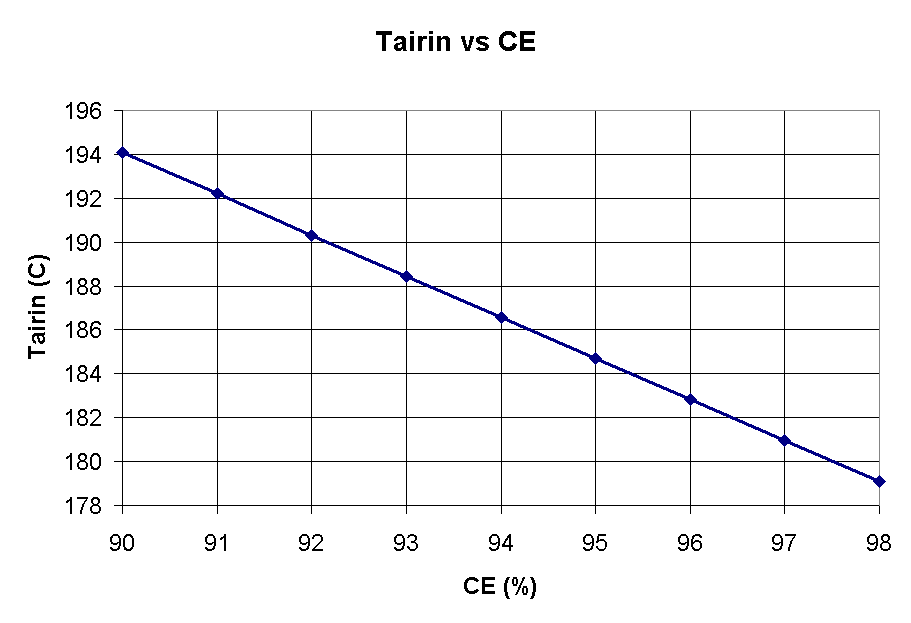
Figure 2 - Tairin vs Current efficiency
Figure 3 shows, as expected that the temperature under the hood will increase with increasing heat off the top of the cell.
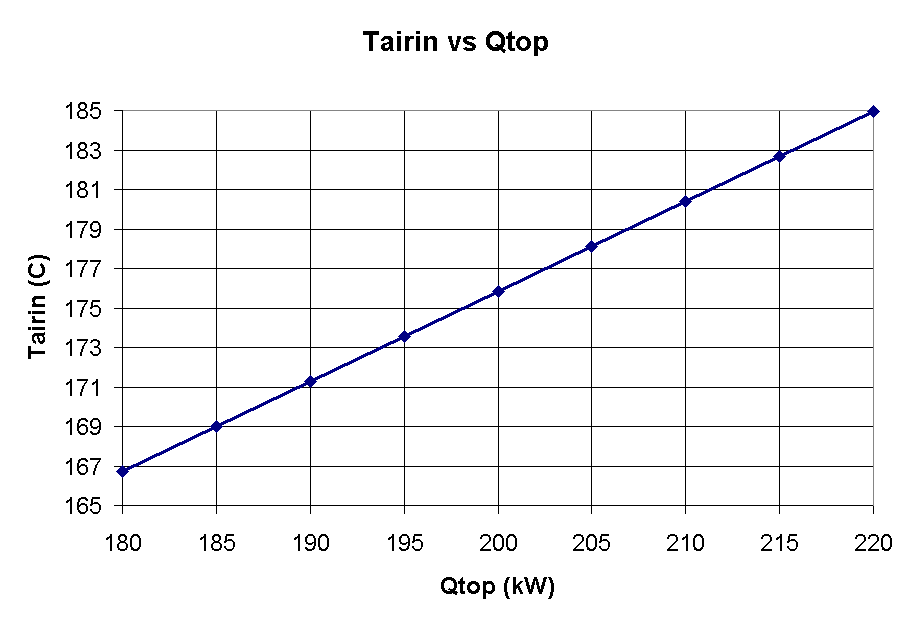
Figure 3 - Tairin vs Sum of heat loss from ore covered and anode stubs
Figure 4 shows that similarly to the current efficiency, the amount of anode carbon that air burns has a non-negligible impact on the temperature under the hood.
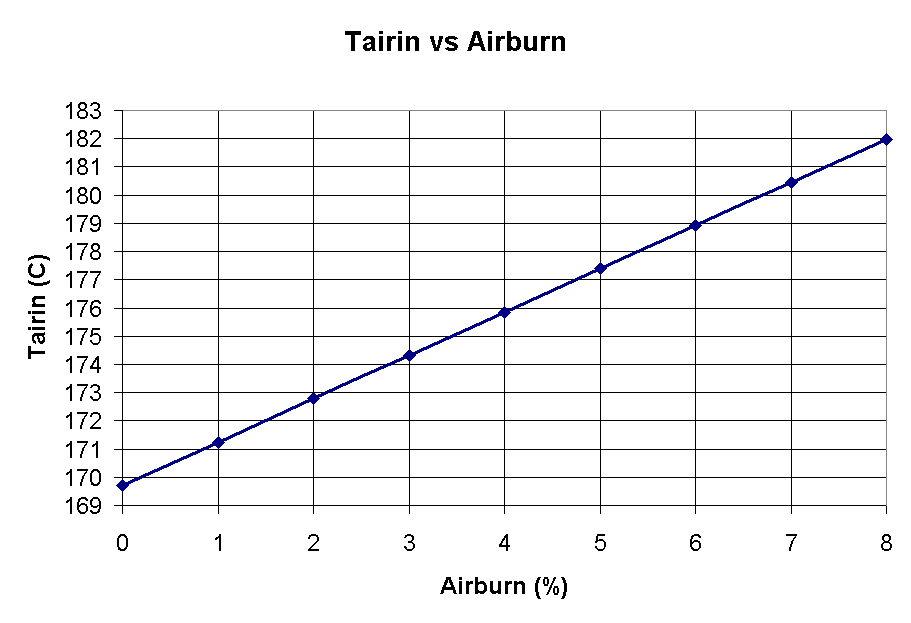
Figure 4 - Tairin vs Anode carbon air burn
REFERENCES